Inorganic material structures with excellent properties have attracted wide research interest in fast and long-life energy storage devices. As one of the classical and most investigated oxides, nanostructured TiO2 has been extensively studied as an anode material in sodium-ion batteries (SIBs) due to its structural stability, safe work-voltage, and low-cost. One-dimensional (1D) TiO2 nanotubes have attracted significant attention owing to the potential properties of large surface area, fast electron/ion transport, and short ion diffusion path. In order to fully take advantages of 1D TiO2 nanotubes in ultra-fast charging sodium ion batteries, it is necessary to design novel structures and simple preparation methods.
Recently, Prof. Zhao Naiqin from Tianjin University and Prof. Qiao Shizhang from the University of Adelaide, Australia, developed a simple gel derivation method. They first synthesized 1D elongated sub-nanotubes (TiO2 SNTs) structure with a high aspect ratio and an open interior structure. TiO2 SNTs consists of anatase/bronze TiO2 nanocrystalline walls with excellent electron/ion transport and reaction kinetics. Consequently, the TiO2 SNTs show great promise for application in SIBs, with a long-life performance with a capacity of 107 mAh g-1 at 16 C after 4000 cycles and a high-rate capacity of 94 mAh g-1 at 32 C. The performance exhibited by this novel nanostructure demonstrates great application potential in ultra-fast and long-life rechargeable batteries. The results were recently published in the international journal “Advanced Materials” (Impact Factor: 21.950).
The main contents of the results are as follows: Firstly, a 1D elongated nanoribbon structure with a width of 80 nm, a thickness of 13 nm and a length of several tens of micrometers was designed and synthesized by the sol-gel method (Figure 1a-c); Then after a simple calcination process at 400 °C, the nanoribbon structures turned into sub-nanotubes (Figure 1d) with a length of tens of microns, a diameter of 25 nm, and a thickness of 7.8 nm (Figure 2a-c).
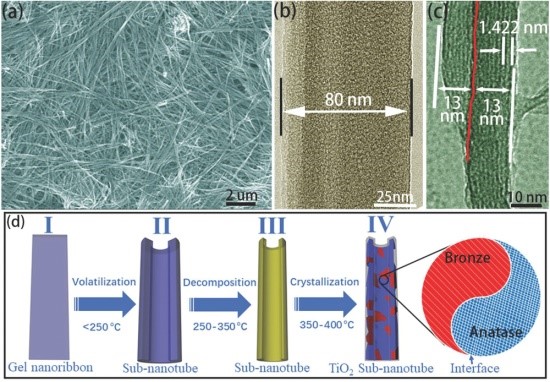
Figure 1. a) SEM image, b) TEM image, c) HRTEM image of CH3COO-[Ti-O-Ti]-OBu gel. and d) Schematic diagram of preparation of gel-transformed TiO2-SNT.
The HRTEM image (Figure 2d) shows that the TiO2 SNTs consist of a significantly large number of small nanocrystals with abundant interfaces. The unique structure of 1D TiO2 sub-nanotubes is responsible for the enhanced Na+ storage performance: The high aspect-ratio and open interior contribute to high specific surface area and electron/ion transport properties; The anatase/bronze TiO2 nanocrystal wall with abundant interfaces further enhance electron/ion diffusion properties and sodium ion storage sites. This results in TiO2 SNTs with superior high-rate performance and long-life performance for promising anode material for SIBs (Figure 3).

Figure 2. a) SEM image, b) TEM image and corresponding SAED pattern, c, d) HRTEM image of TiO2 SNT. (The illustration in (b) is a schematic view of the cross section of the nanotube; h, R and α in (b) represent the thickness of SNT wall, the radius of outer SNT and the rolling curvature, respectively; the lines in A, B and (d) respectively represents anatase, bronze and microcrystalline interfaces.
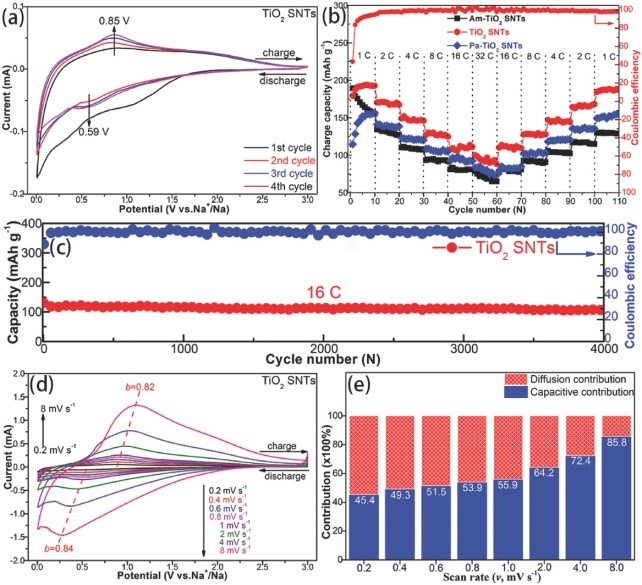
Figure 3. Electrochemical properties of TiO2 SNT for Na half cells: a) CV curve. b) Rate properties of Am-TiO2 SNT, TiO2 SNT and Pa-TiO2 SNT. c) Long life cycle performance at 16 C. d) CV curves at different rates and corresponding e) capacitance contributions. 1 C = 335 mAh g-1
The first author of this paper is Mr. Chen Biao, a doctoral student jointly trained by Tianjin University and the University of Adelaide. The corresponding authors are Prof. Zhao Naiqin and Prof. Qiao Shizhang. The research was supported by the National Natural Science Foundation of China, the Australian Research Council, and the China Scholarship Council.
Title: 1D Sub-Nanotubes with Anatase/Bronze TiO2 Nanocrystal Wall for High-Rate and Long-Life Sodium-Ion Batteries
Literature link: https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201804116
By: School of Materials Science and Engineering
Editor: Qin Mian and Keith Harrington






